Products
N-Methylurea
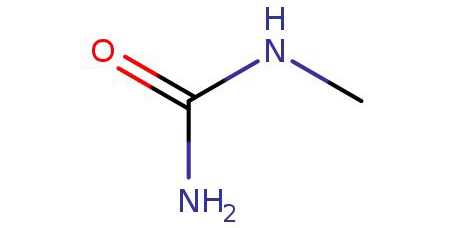
Synonyms:methylurea;monomethylurea
Chemical Property of Methylurea
● Appearance/Colour:White, crystalline needles.
● Vapor Pressure:19.8mmHg at 25°C
● Melting Point:~93 °C
● Refractive Index:1.432
● Boiling Point:114.6 °C at 760 mmHg
● PKA:14.38±0.46(Predicted)
● Flash Point:23.1 °C
● PSA:55.12000
● Density:1.041 g/cm3
● LogP:0.37570
● Storage Temp.:Store below +30°C.
● Solubility.:1000g/l (Lit.)
● Water Solubility.:1000 g/L (20 ºC)
● XLogP3:-1.4
● Hydrogen Bond Donor Count:2
● Hydrogen Bond Acceptor Count:1
● Rotatable Bond Count:0
● Exact Mass:74.048012819
● Heavy Atom Count:5
● Complexity:42.9
Purity/Quality
99% *data from raw suppliers
N-Methylurea *data from reagent suppliers
Useful
● Chemical Classes: Nitrogen Compounds -> Urea Compounds
● Canonical SMILES: CNC(=O)N
● Uses: N-Methylurea is used as a reagent in the synthesis of bis(aryl)(hydroxyalkyl)(methyl)glycoluril derivatives and is a potential byproduct of caffeine.
Methylurea, also known as N-methylurea, is a chemical compound with the molecular formula CH4N2O. It is an organic compound belonging to the class of urea derivatives. Methylurea is derived from urea by substituting one of the hydrogen atoms with a methyl group (-CH3).Methylurea is commonly used in organic synthesis as a reagent or building block in various chemical reactions. It can serve as a source of the carbonyl group (-C=O) or the amino group (-NH2) in a variety of synthetic transformations. Methylurea is also used in the production of pharmaceuticals, agrochemicals, and dyes.It is important to handle methylurea with caution, as it may be toxic if ingested or if significant dermal exposure occurs.